Assuming that the specific heat is constant during the entire process, the final temperature of the two blocks must be
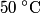 in order for energy to be conserved. The amount of energy required to increase the temperature of the block initially at in order for energy to be conserved. The amount of energy required to increase the temperature of the block initially at 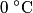 to to 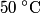 is is 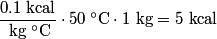 The blocks are enclosed in a perfectly insulating container, so this must also be the amount of energy transferred between the two blocks. Therefore, answer (D) is correct. |